Achieving smooth and dendrite-free lithium deposition is critical for the commercialization of lithium metal batteries (LMBs). This study explores the role of silver salts (AgNO3, AgF, AgCl, Ag2CO3, and Ag2SO4) in regulating lithium nucleation and solid–electrolyte interphase (SEI) stability. Upon in situ reduction under lithiation conditions, silver ions form a uniform metallic Ag layer that serves as a highly lithiophilic nucleation seed, significantly lowering the plating overpotential and promoting dense lithium growth. Concurrently, the dissociated anions contribute to SEI formation, influencing interfacial stability and lithium-ion transport. Electrochemical characterizations reveal that AgNO3-coated substrate achieves near-100% Coulombic efficiency, the lowest nucleation overpotential, and superior cycling stability compared to other silver salts. Advanced lithium-sensitive energy-dispersive X-ray spectroscopy mapping confirms the uniform distribution of lithium and silver, correlating with the enhanced lithium plating reversibility. X-ray photoelectron spectroscopy and X-ray diffraction further validate the formation of a stable Li–Ag alloy and a Li3N-enriched SEI, which collectively suppress dendrite formation and improve interfacial stability. These findings establish AgNO3-coated current collector as a promising and scalable strategy for high-performance LMBs, providing new insights into interface engineering for next-generation energy storage systems.


The development of high-performance lithium‒sulfur batteries (LSBs) has been focused on overcoming the limitations associated with traditional polysulfide catholyte synthesis. We report an innovative catholyte synthesis method using lithium-arene complexes, offering significant advancements in terms of solubility, stability, and scalability. By leveraging the interaction of metallic lithium with biphenyl (BP) and sulfur, we develop a Li+BP+S catholyte formulation that outperforms conventional Li2S+S systems. The Li+BP+S catholyte demonstrates superior solubility, achieving up to 12 M active sulfur and faster dissolution rates at lower temperatures, reducing preparation times by 66%. Electrochemical evaluations revealed enhanced capacity retention, with the catholyte maintaining 83.2% of its initial capacity after 500 cycles and exhibiting minimal capacity fading of 0.03% per cycle. Material characterization confirmed a uniform sulfur distribution, improved charge transfer capability, and reduced polysulfide clustering, as evidenced by NMR, SEM, and XPS analyses. The Li+BP+S system also demonstrated high-rate capability and long-term stability, retaining significant capacity under lean electrolyte conditions. The mechanism by which the addition of arenes aides Li dissolution is also proposed on the basis of theoretical calculations. These findings highlight the potential of lithium–arene complexes to revolutionize LSB technology, paving the way for safer, more efficient, and scalable LSB systems.
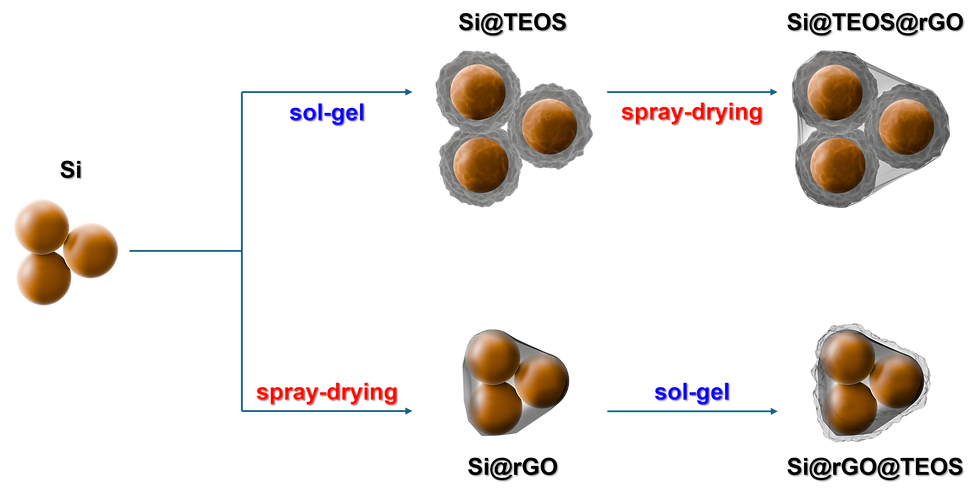
This study explores the optimization of double-shelled silicon (Si) composite anodes for lithium-ion batteries (LIBs) by examining the coating sequence of tetraethyl orthosilicate (TEOS) and reduced graphene oxide (rGO). The Si55@TEOS29@rGO16 anode, which features an inner TEOS-derived layer and outer rGO shell, exhibits an initial capacity of 1763 mA h g−1 at 0.1C and maintains 1153 mA h g⁻¹ after 300 cycles at 0.5C, achieving a capacity retention of 85.5% when incorporating the trimethylsilyl phosphate (TMSPi) electrolyte additive. The performance improvement is attributed to the hierarchical structure, where the porous SiO2 layer effectively passivates the Si core, while the rGO shell enhances conductivity and structural stability. Comparative analysis reveals that the optimized coating sequence and TEOS content significantly enhance cycling stability, with the Si55@TEOS29@rGO16 anode surpassing other configurations in specific capacity after 129 cycles. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) analyses confirm the uniform coating and structure stability after cycling. Electrochemical impedance spectroscopy (EIS) shows reduced impedance, and cyclic voltammetry (CV) indicates stable lithiation/delithiation processes. The inclusion of the TMSPi additive further stabilizes the solid electrolyte interphase (SEI) and inhibits capacity fading during initial cycles. This study demonstrates that optimizing the coating sequence, SiO2 content, and electrolyte additives can significantly improve the electrochemical performance and durability of Si-based anodes, positioning them as strong candidates for next-generation LIBs.








































